Back to Projects List
Automatic multi-anatomical skull structure segmentation of cone-beam computed tomography scans using 3D UNETR
- Maxime Gillot (UoM)
- Baptiste Baquero (UoM)
- Celia Le (UoM)
- Romain Deleat-Besson (UoM)
- Jonas Bianchi (UoM, UoP)
- Antonio Ruellas (UoM)
- Marcela Gurge (UoM)
- Marilia Yatabe (UoM)
- Najla Al Turkestani (UoM)
- Kayvan Najarian (UoM)
- Reza Soroushmehr (UoM)
- Steve Pieper (ISOMICS)
- Ron Kikinis (Harvard Medical School)
- Beatriz Paniagua (Kitware)
- Jonathan Gryak (UoM)
- Marcos Ioshida (UoM)
- Camila Massaro (UoM)
- Liliane Gomes (UoM)
- Heesoo Oh (UoP)
- Karine Evangelista (UoM)
- Cauby Chaves Jr
- Daniela Garib
- F ́abio Costa (UoM)
- Erika Benavides (UoM)
- Fabiana Soki (UoM)
- Jean-Christophe Fillion-Robin (Kitware)
- Hina Joshi (UoNC)
- Lucia Cevidanes (UoM)
- Juan Prieto (UoNC)
The segmentation of medical and dental images is a fundamental step in automated clinical decision support systems. It supports the entire clinical workflow from diagnosis, therapy planning, intervention, and follow-up. In this paper, we propose a novel tool to accurately process a full-face segmentation in about 5 minutes that would otherwise require an average of 7h of manual work by experienced clinicians. This work focuses on the integration of the state-of-the-art UNEt TRansformers (UNETR) of the Medical Open Network for Artificial Intelligence (MONAI) framework. We trained and tested our models using 618 de-identified Cone-Beam Computed Tomography (CBCT) volumetric images of the head acquired with several parameters from different centers for a generalized clinical application. Our results on a 5-fold cross-validation showed high accuracy and robustness with an Dice up to 0.962 pm 0.02.
- Create only one model for multiple structures.
- Create a slicer module for the algorithm
- Add new structure to segment
- Deploy the AMASSS tool with the updated trained models
- Get the data merged by the clinicians for the skull.
- Use the begening of a slicer module to create a new one for AMASSS.
- Use new dataset to train new HD models.
- An algorithm has already been made to run segmentation out of slicer as a docker to implement in the DSCI
- We collected data to generate segmentation model using the MONAI librairie
- For large field of view :
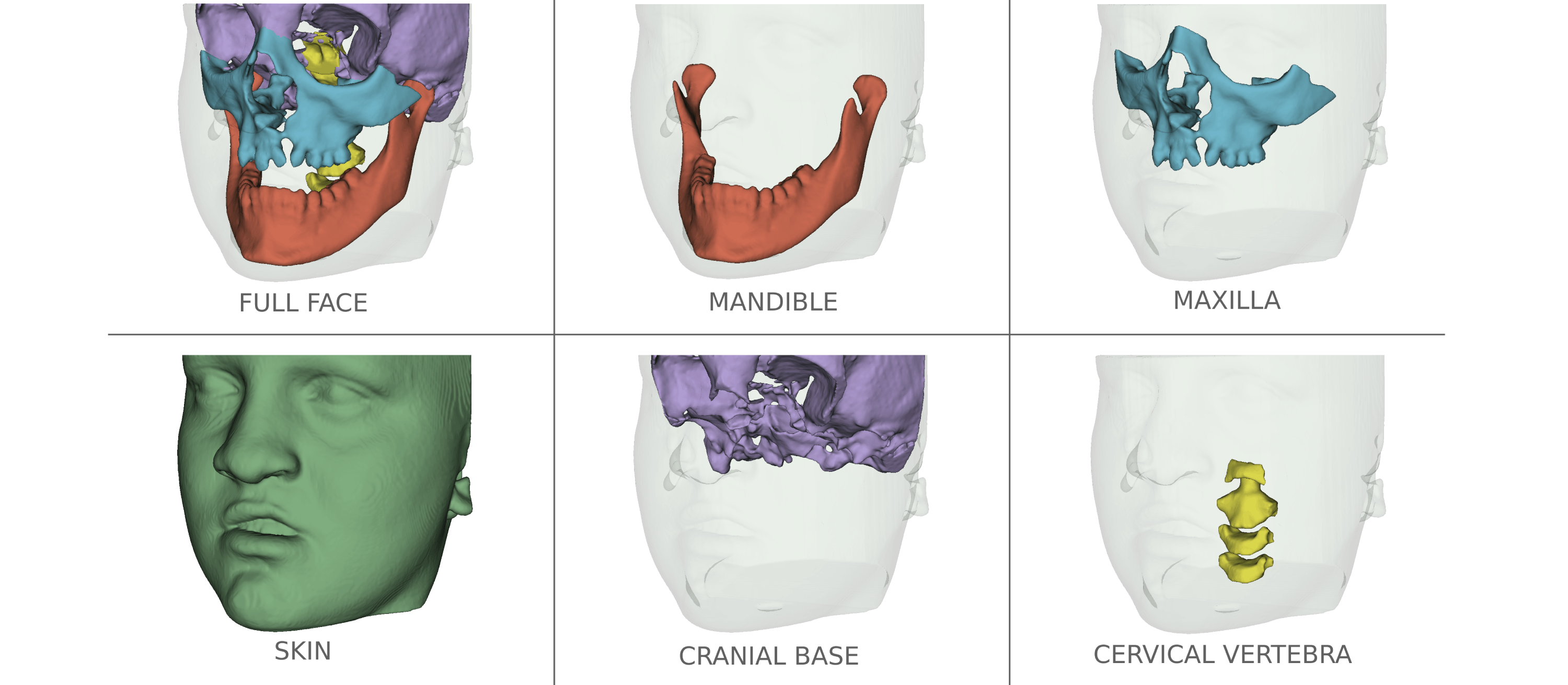
- A model has been trained to generate a segmentation of 5 skull structures (mandible, maxilla, cranial base, cervical vertebra and upper airway)
- An other to segment the skin.
- For small field of view :
- A model for upper and lower root canal has been trained as well as HD mandible and maxilla
- We still need data to train networks for crown and mandible canal segmentation
-
To be more user friendly, the development of an AMASSS module for Slicer has been started in march.
-
The UI of a slicer module was already started befor project week and has now been updated.
-
We linked the UI with a CLI module to run the prediction/segmentation directly on the user computer through Slicer 5's python 3.9
-
The module has been tested locally with clinicians and is ready to be deployed as a Slicer module as a part of the slicer CMF extention ( The code is available at https://github.com/Maxlo24/Slicer_Automatic_Tools )
-
We colaborated with Slicer Batch Annonymize (Hina Shah, Juan Carolos Prieto) to use AMASSS as a first step to perform defacing of patients scans during the batch anonymisation process. ( Figure 3 Mask for defacing )
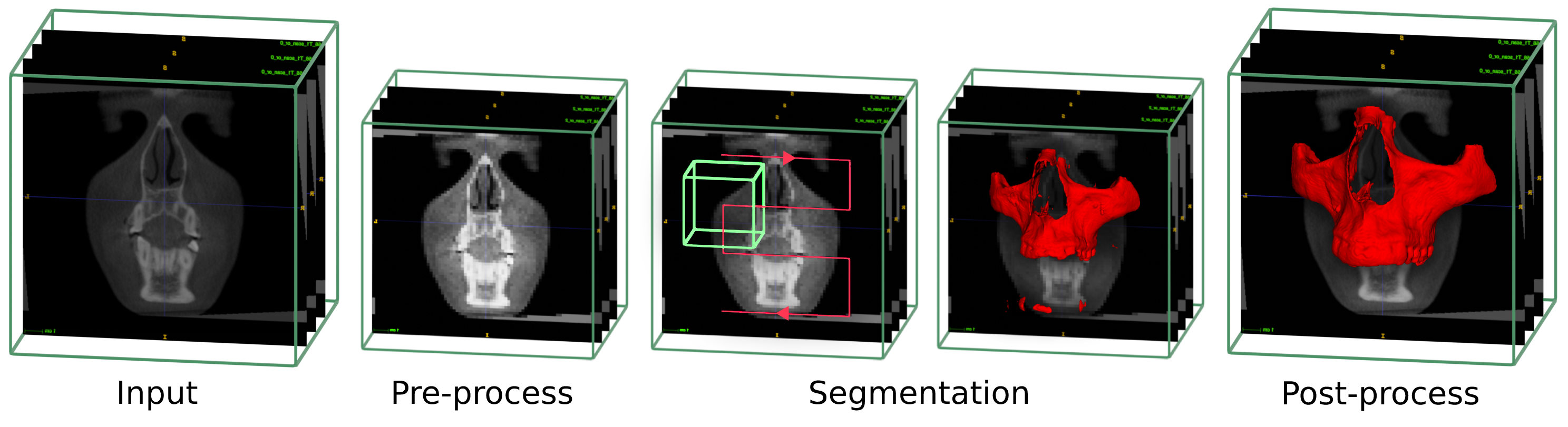
- Contrast correction and rescaling to the trained model spacing
- Use the UNETR classifier network through the scan to perform a first raw segmentation
- Post process steps to clean and smooth the segmentation
- Upscale to the original images size
-
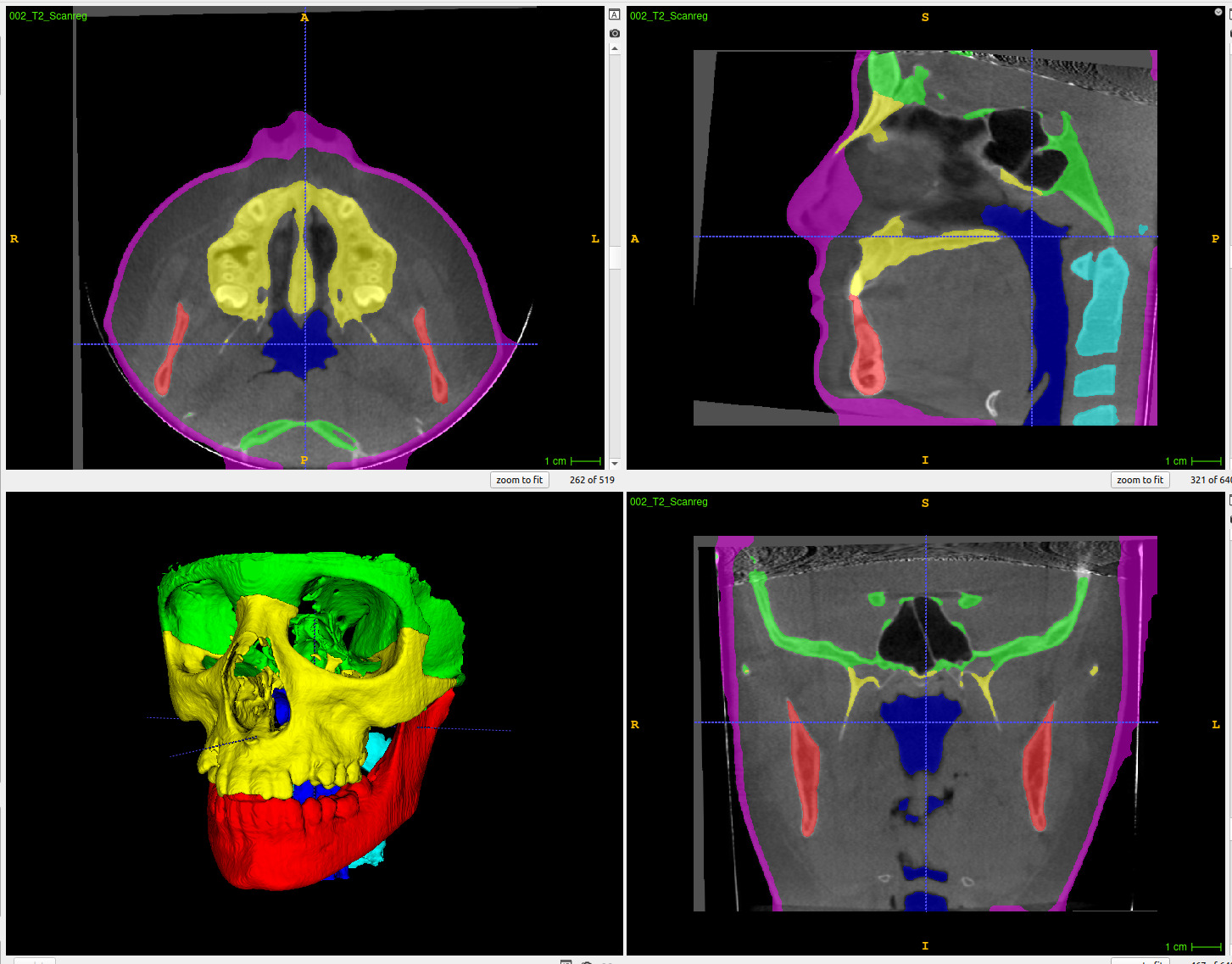
Selection of the different parameters and which structure to segment
-
Use of a dialog progress bar to show/cancel the progress of the segmentation in real time (top right end corner).
-
One the 3D view, result of one of the segmentation with the generated VTK files
-
A prediction takes from 120s to 300s for one patient depending on the local computer GPU capacity ( 15GB down to 3GB)
- The scan intensity in the pink region ( mainely nose, lips and eyes ) will be set to 0 to make it impossible to identify the patient
- The bones segmentations are used to make sure we dont remove important informations during the process